Abstract
Introduction Diffuse large B cell lymphoma (DLBCL) patients who are refractory or relapse (r/r) within 12 months of completion of initial therapy (refractory and early relapse, RER) have inferior outcomes than those relapsing 12 months or after (late relapse, LR). Recent trials of anti-CD19 chimeric antigen receptor T (CAR-T) cell therapy have demonstrated improved event-free survival (EFS) in RER-DLBCL when compared with standard chemotherapy with intent to undergo autologous stem cell transplant (ASCT). Due to clinical urgency and logistic factors many patients with r/r DLBCL will receive second line systemic chemotherapy. The optimal treatment for those responding to second line therapy is not yet well defined.
Methods We conducted an IRB approved retrospective review of r/r DLBCL patients treated with rituximab, ifosfamide, carboplatin, etoposide (R-ICE) at three academic medical centers between 2010-2020. Baseline characteristics and outcomes were collected from the electronic medical record. Patients having RER-DLBCL were defined as those who had disease that was r/r within 12 months of the end of initial therapy. Outcomes evaluated included disease response to initial and second line therapy, subsequent ASCT and CAR T cell therapy, progression free survival (PFS) and overall survival (OS). Response was assigned by CT scan in 38 (13%) and by PET in 236 (81%) of patients. All analyses were done using R and its packages.
Results We identified 291 patients treated with R-ICE for r/r DLBCL, 182 (62.5%) of whom had RER-DLBCL and 109 (37.4%) and LR-DLBCL. Baseline characteristics between RER and LR-DLBCL were comparable (Table 1), with the exception of age. RER-DLBCL patients received a median of 2 cycles vs. 3 cycles of RICE for LR-DLBCL (p = 0.008). Patients with RER-DLBCL had overall (OR) and complete response (CR) rates of 62% and 30%, vs. 86% and 45% among patients with LR-DLBCL, respectively (p < 0.0001). 170 patients underwent ASCT. 79 patients received CAR T cell therapy, 42 (14%) without prior ASCT and 37 (13%) after ASCT.
After a median follow up of 53 months (IQR, 36-76), median PFS for ER-DLBCL vs. LR-DLPBCL was 6 months vs. 31 months (HR=1.84 [95%CI 1.36-2.49, p < 0.001), with 4-year PFS estimates of 24% (95% CI 18-32%) vs. 40% (95% CI 31-51%), respectively. Median OS was 36 months for ER-DLBCL vs. 68 months for LP-DLBCL (HR=1.63 [95% CI 1.13-2.36], p = 0.009), with 4-year estimates of 48% (95% CI 40-56%) vs. 66% (95% CI 57-76%), respectively.
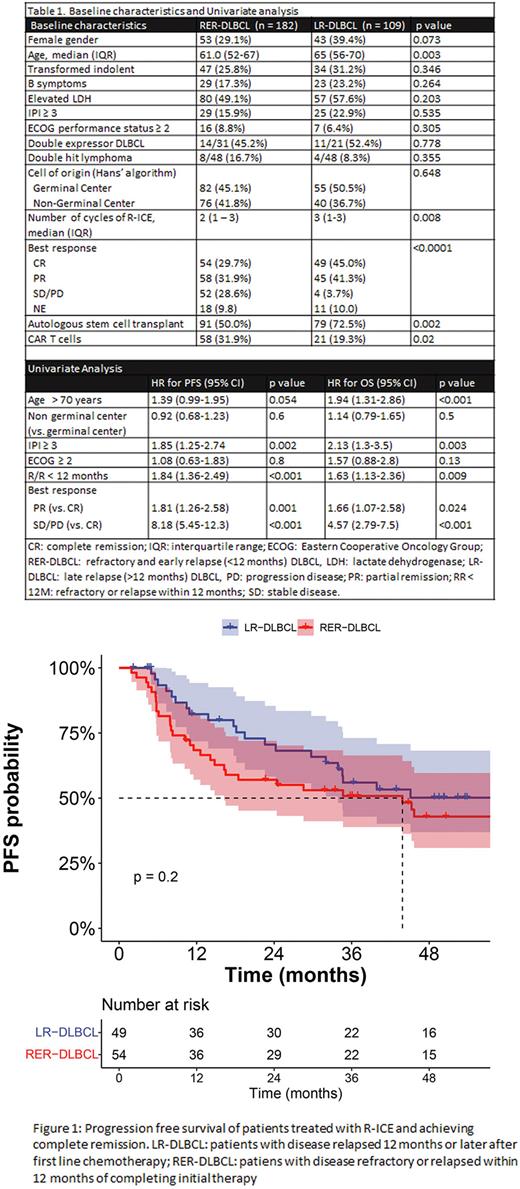
Among patients achieving CR after R-ICE, median PFS was 43 months for RER-DLBCL vs. not reached for LR-DLBCL (HR=1.43 [95% CI 0.8-2.4], p = 0.2) (Figure 1), with 4-year PFS estimates of 43% (95% CI 31 - 60%) and 50% (95% CI 37 - 68%), respectively. Median OS for patients in CR after R-ICE was 85 months for RER-DLBCL and 102 months for LR-DLBCL (HR = 0.98 [95% CI 0.5 - 1.92], p > 0.9), with 4-year OS estimates of 69% (95% CI 57 - 84%) and 72% (95% CI 60 - 87%), respectively.
Patients who underwent ASCT after achieving CR had median PFS of 71 months (95% CI 29 - NR), while median survival was not reached (95% CI 85 months - NR). Four-year PFS and OS estimates of 53% and 77%, respectively. Among patients undergoing ASCT after CR, there was no statistically significant difference in PFS (4-year PFS: RER-DLBCL 48.5% vs. LR-DLBCL 58.6%, p = 0.1) or OS (4-year OS: RER-DLBCL 78.2% vs. LR-DLBCL 74.9%, p = 0.9)
On univariate analysis, elevated LDH, RER-DLBCL and failure to achieve CR after R-ICE (i.e. PR or SD/PD) were associated with increased hazards of progression, while age > 70 years, elevated LDH, RER-DLBCL and failure to achieve CR were associated with increased hazards of death (Table 1). On multivariate analysis, however, the effect of early relapse was not statistically significant for risk of progression or death, whereas response to R-ICE retained statistical significance.
Conclusions The poor outcomes of RER-DLBCL are the result of a high proportion of patients with chemorefractory disease. However, DLBCL patients who achieve CR after R-ICE salvage can achieve long term disease control, regardless of the time to relapse from initial therapy, in particular if they proceed to ASCT. These results suggest second line chemotherapy, followed by ASCT vs. CAR T for chemosensitive and chemorefractory patients may be a response - adapted approach that can maximize DLBCL patient outcomes, regardless of time to relapse.
Disclosures
Caimi:Kite: Consultancy; Genentech: Consultancy; Incyte: Consultancy; Novartis: Consultancy; MEI Pharma: Honoraria; Janssen: Consultancy; ADC Therapeutics: Honoraria, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; GenMab: Honoraria; BMS: Honoraria. Hill:Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding.
Author notes
*Asterisk with author names denotes non-ASH members.